Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na. Sodium is a soft, silvery-white, highly reactive metal. The Sodium Zeeman Effect The sodium spectrum is dominated by the bright doublet known as the Sodium D-lines at 588.9950 and 589.5924 nanometers. From the energy level diagram it can be seen that these lines are emitted in a transition from the 3p to the 3s levels. The sodium doublet is further spit by the application of an external magnetic field (.
- Sodium Electrons And Neutrons
- Number Of Valence Electrons Sodium
- Sodium Electrons Gained Or Lost
- Sodium Electrons And Valence Electrons
How many valence electrons does sodium have?
3 Answers
Sodium, like all the group 1 alkali metals, has one valence electron.
Explanation:
Paint brush thick. Valence electrons are the outermost electrons, and are the ones involved in bonding. Sodium has 11 electrons: its atomic number is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons.
Electrons are arranged in 'shells' or energy levels. Depending on your level of Chemistry, it is probably easier to think of them as particles orbiting the nucleus. The first 'shell' can have 2 electrons. The second 'shell' can have up to 8 electrons. The third 'shell' is a bit more complicated but let's just say that it takes up to 8 electrons as well (for now..).
So, sodium's 11 electrons are arranged this way: 2 electrons in the first 'shell', 8 electrons in the second 'shell'; and 1 electron (the valence electron) in the third 'shell'. We write this as 2.8.1. The last number is how we know the number of valence electrons.
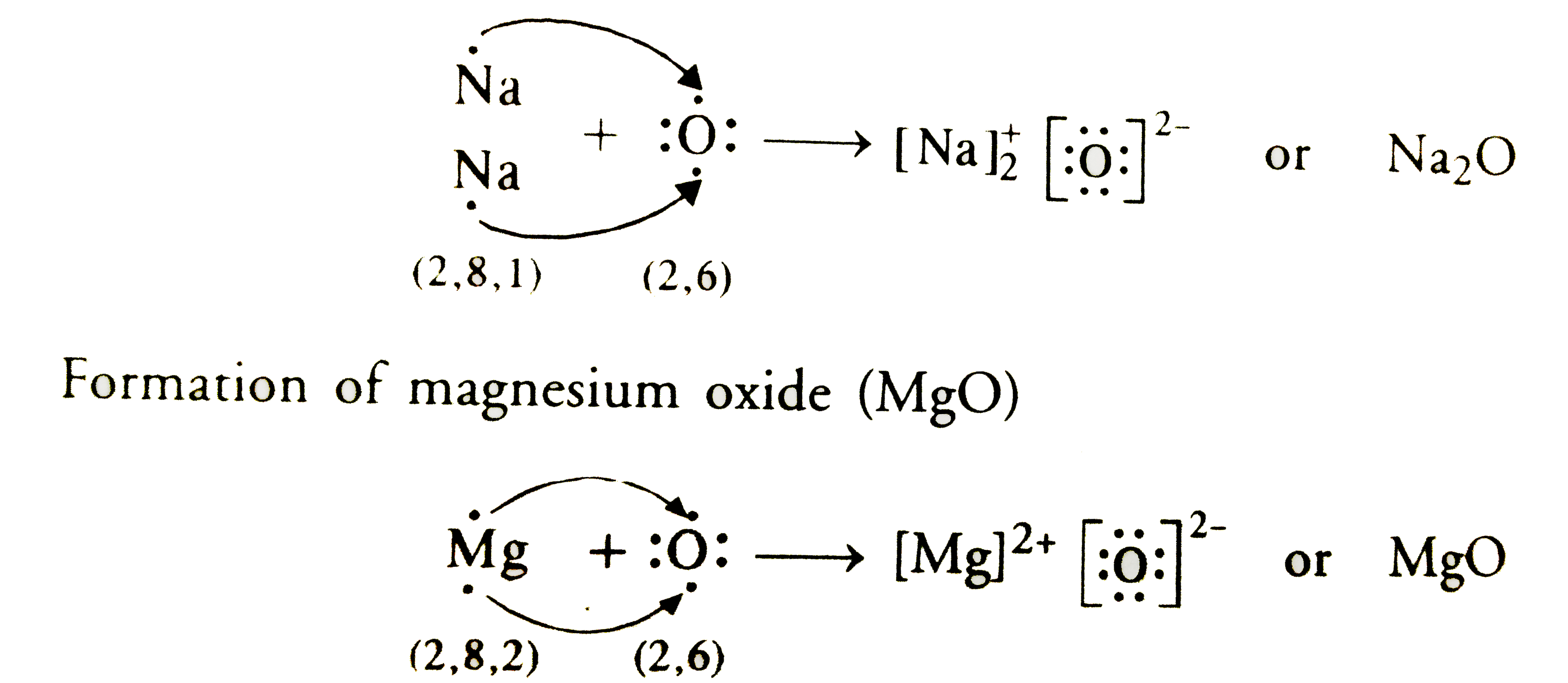
Aluminium has the electron arrangement 2.8.3. It has 3 valence electrons. Fluorine has the electron arrangement 2.7. It has 7 valence electrons. This trend only gets broken at Sc (#21).
Here is a video which gives further explanation of this topic.
video from: Noel Pauller
Sodium Electrons And Neutrons
Sodium has one valence electron. Valence electrons are electrons found in the outermost shell of an atom. The shell number representing the valence shell will differ depending on the atom in question. For sodium, which is in the 3rd row of the periodic table, the valence electrons will be found in the 3rd shell. For fluorine, which is in the second row, the valence electrons will be found in the second shell. (Quick note: In the periodic table, rows are horizontal lines, rows are vertical lines.)
Photozoom pro. There are a number of ways to approach this question. The easiest way is to use the periodic table as your guide. If we start by looking at the second row, and follow along horizontally, we can assign valence electron numbers to each element. Starting with lithium (Li), which has one valence electron, we can move across horizontally and add one valence electron each time as follows, Bohemian rhapsody guitar pro tab.
Number Of Valence Electrons Sodium
Element : Number of Valence Electrons
Li : 1
Be : 2
B : 3
C : 4
N : 5
O : 6
F : 7
Ne : 8 (or zero)


Furthermore, every atom on the periodic table below lithium (Li) will also have one valence electron (i.e. Na, K, Rb, Cs, Fr). The same goes for every other element listed, as well. For instance, Cl, Br, I and At will each have seven valence electrons like fluorine (F). Don't worry too much about elements found in between the Be column and the B column. For the so-called transition metals, valence electrons are not as well defined.

Sodium Electrons Gained Or Lost
Explanation:
Valence electrons are found in the highest energy s and p orbitals in the main group of elements. The electron configuration for a neutral sodium is
Sodium Electrons And Valence Electrons
Actually, all of the elements in Group1/IA have one valence electron.
Related questions
